bot blogger
a botanical blogger
Friday, 17 March 2017
Sunday, 21 February 2016
Note on the identity of Salacia vellaniana Udayan, Yohannan & Pradeep (CELASTRACEAE)
RESEARCH ARTICLE
Note on the identity
of Salacia vellaniana Udayan, Yohannan & Pradeep
(CELASTRACEAE)
N. Sasidharan and K. Muhammad Anaz
Kerala Forest Research Institute, Peechi, Thrissur 680 653, Kerala
Abstract
N. Sasidharan and K. Muhammad Anaz
Kerala Forest Research Institute, Peechi, Thrissur 680 653, Kerala
Abstract
INTRODUCTION
Gamble (1916) described 3 new species of Salacia viz. S. malabarica, based on the collections of TF Bourdillon from ‘Colatoorpolay, Travancore’; S. beddomei on the collections of RH Beddome from Anamalays and S. talbotii on the collections of WA Talbot from North Kanara. Whiting & Kaul (1940) renamed Salacia talbotii Gamble as S. gambleana as the former name is a later homonym of S. talbotii Baker f. (1913). Udayan et al., (2013) described S.vellaniana as a new species from Vellanimala, Thrissur District of Kerala. Sasidharan and Sivarajan (1996) identified similar specimens from Vellanimala, Thrissur as S. macrosperma based on the fimbriate calyx lobes, entire leaf margins and nearly smooth fruit surface. In the light of description of S. vellaniana, we made a critical study of the specimens from Vellanimala, Thrissur with the species of Salacia so far described from Peninsular
India. It is found that S. vellaniana shares many characters with S. gambleana. Gamble (1916) differentiated S.talbotii from S. macrosperma based on the smaller oblong coriaceous leaves with entire margin, 4-6 mm long petioles, 1-8 flowered cymes (against 22-30 flowered cymes), 3 or 4 stamens and pale reddish-orange echinate fruits.
Examination of the protologue and images of the type specimens of S. talbotii (WA Talbot 1361, WA Talbot 1217, 2.6.1885, North Kanara) it is found that S. vellaniana is very much matching with S. gambleana.
Udayan et al., (2013) described the petals as ovate-lanceolate, but it is not reflected in the illustration. In fact the petals are ovate-oblong with obtuse apex. The filaments are flattened and tapering towards apex and reflexed as described by Gamble. The ovary of S. vellaniana was descried as 3-loculed with 1 ovule in each locule and fruits
globose or obovate, tuberculate, pale reddish-orange; seeds 3. Observation of fresh specimens from the type locality (Vellanimala), reveal that there are 2 ovules in each locule and up to 5 seeds in the fruits, further endorsing the similarity with S. gambleana. The fruit surface varies from nearly smooth with 1 or 2 warts to completely rugose with linear ridges. Thus, there are no true diagnostic characters to distinguish S. vellaniana from S. gambleana. It can be confirmed that Salacia vellaniana was erected based on imperfect study of materials by the authors
Therefore, it is reduced to a synonym of S. gamblei Whiting & Kaul. Description based type specimens and our fresh collections are provided along with images.
Salacia gambleana
Whiting & Kaul, Bull. Misc. Inf. Kew. 1940:300.1940; Ramamurthy & Naithani in NP Singh et al., (eds.) Flora India 5:153.2000; Punetkar & Lakshminarasimhan, Fl. Anshi National Park 153.2011. Salacia talbotii Gamble, Bull. Misc. Inf. Kew. 1916:133.1916 (non EG Baker, 1913); Saldanha & Singh in Saldanha Fl. Karnataka 2:92.1996.
Salacia vellaniana Udayan, Yohannan & Pradeep, Candollea 68: 148. 2013. syn. nov.
Scandent lianas; young branchlets brownish, often lenticellate. Leaves opposite to sub-opposite, 6-11 x 2.5-3.5 cm, oblong, apex acute to acuminate, base cuneate or attenuate, margin entire or remotely crenate, subcoriaceous; lateral nerves 5 or 6 pairs, reticulations faint; petiole 4-6 mm long. Flowers in simple fascicles of 2-8, from axillary or extra-axillary tubercles; pedicel 4-6 mm long. Calyx lobes 5, triangular ovate, green, ca. 1 mm long, margin fimbriate. Petals 5, 1.8-2 x 1-1.2 mm, ovate-oblong with a small notch towards the apex, obtuse, erect, green with a tinge of yellow towards the upper margins. Disk green turning creamy yellow, 1 x 1.5 mm, oblong, tapering towards apex, completely covering the ovary. Stamens 3, rarely 4, discoid, 0.2 mm long, creamy-white with a brown tinge, arising from within the disk; filaments flattened, recurved, creamy-white when young, become yellow with orange tinge later. Ovary 3-loculed; ovules 2 in each locule; stigma simple, pale green. Fruits globose or obovoid, 3-4.5 x 2.5-3.5 cm, rind 2-2.5 mm thick, smooth to tuberculate, with thickened linear ridges, pale reddish-orange; seeds 2-5,
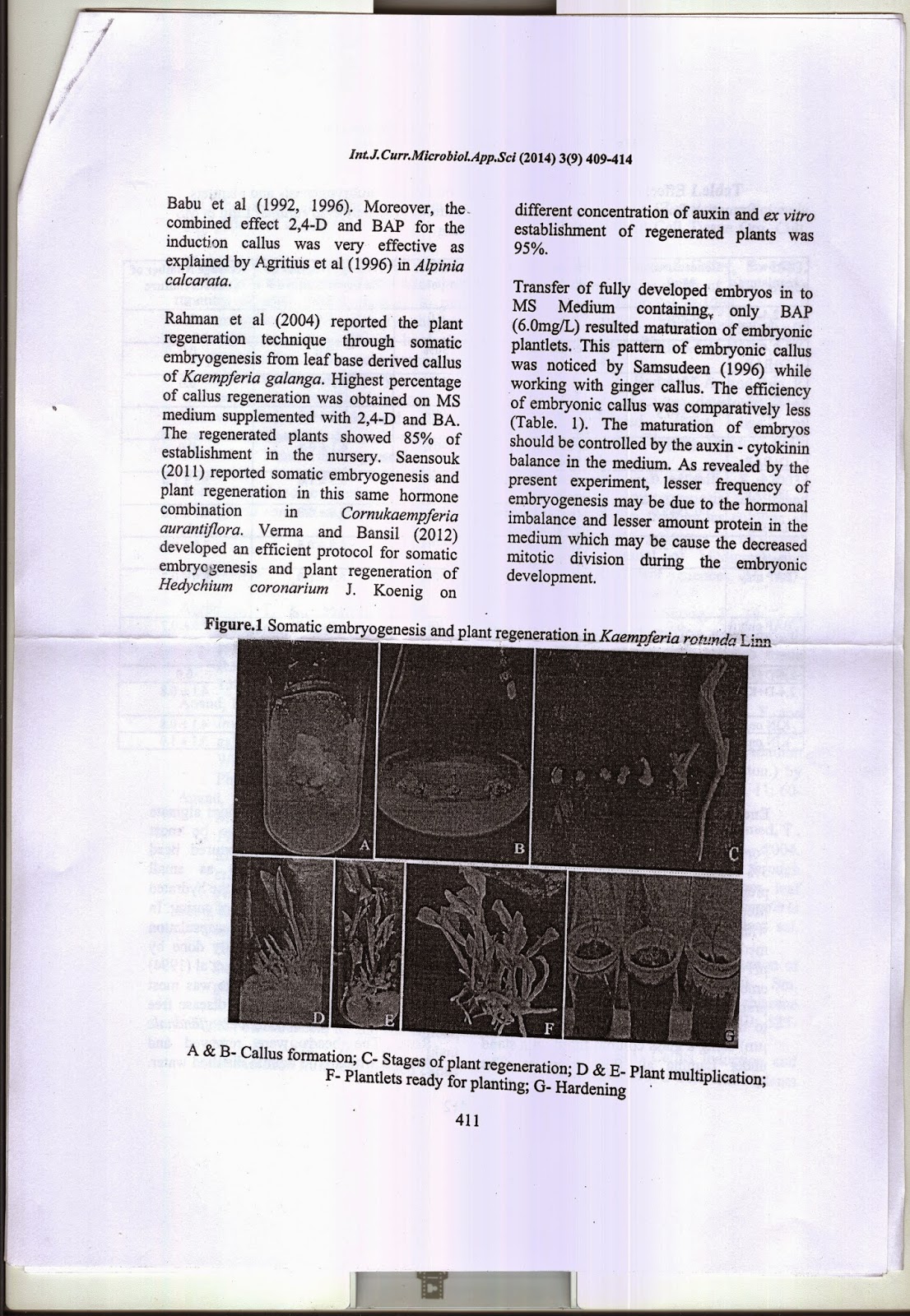
ellipsoid, slightly angular, immersed in pulp, 1.5-2 x 1-1.5 cm, brown. [ Plate I].
Distribution: Western Ghats of Kerala and Karnataka
Specimens examined: Maharastra, North Kanara WA Talbot 1361 dated 25.01.1886 ; 1217 dated 02.06.1886 [http://specimens.kew.org/herbarium/ K000669982, K000669983 accession on 19.12.2014 10.45 IST] ; Kerala Thrissur, Vellanimala N. Sasidharan 3079 dated 18.04.1984; 27903 dated 11.01.2013; 3940 dated 13.03.1987; 3484 dated 10.05.1985 [KFRI]; PS Udayan et al., 03371, dated 23.02.2005 ; 06121 dated 20.06.2009 [Herbarium CMPR, Kottakkal].
Acknowledgements
The authors are thankful to Dr. P. Lakshminarasimhan, Scientist, Central National Herbarium, Botanical Survey of India, P.O. Botanic Garden, Howrah, for providing details on the distribution in Karnataka. We are also thankful to Board of Trustees of the Royal Botanic Gardens, Kew, for permitting to use the scanned images of Salacia talbotii type specimens. The first author acknowledges the financial assistance for the study from MoEF, Government of India.
References
Gamble, JS, 1916. Decades Kewensis. Bull. Misc. Inf. Kew 1916: 131-136.
Gamble JS, 1918. Family Hippocrateaceae. In The Flora of the Presidency of Madras. Adlard & Son Ltd., London. Whiting MM and KN Kaul 1940. New or Little Known Plants from Southern India XIII . Bull. Misc. Inf. Kew 1940:300-302.
Sasidharan, N. and V.V. Sivarajan 1996. Flowering Plants of Thrissur Forests. Scientific publishers, Jodhpur. Udayan PS, Regy Yohannan, Devipriya MS, Devipriya V & AK Pradeep, 2013. Salacia vellaniana Udayan,Yohannan & Pradeep (Celastraceae), a new species from India. Candollea 68(1):147-149.
Thursday, 10 December 2015
Saturday, 19 September 2015
ABSTRACT OF Ph. D THESIS OF Dr. ARCHANA CP
Title of Thesis: Scaling up of Microrhizome and Minirhizome Technology for Disease Free Planting Material Production in Ginger and Turmeric
Dr. ARCHANA CP
Ginger (Zingiber officinale Rosc.) and Turmeric
(Curcuma longa L.) belonging to the family Zingiberaceae are
two commercially important medicinal spices. These are rhizomatous
perennials grown as annuals. Ginger is thought to have originated in
the tropical jungles of Asia and commercially grown in India, China,
South- East Asia, West Indies, Mexico, Africa, Fiji and Australia.
Turmeric is a native of Southeast Asia. In ancient India, ginger was
considered as Mahoushadi (great cure). Turmeric, the golden
spice- the dried rhizomes of C. longa, is famed as the spice
of life. It has strong association with socio-cultural life of Indian
subcontinent and was described as ‘herb of sun’ by people of
Vedic period.
The productivity of ginger and turmeric in India is
decreased due to many diseases that affect the quality and quantity
of the rhizome produced. In field, ginger and turmeric are prone to a
number of pathogens and diseases of soil-borne and rhizome-borne
nature. The success of these crops mainly depends on the use of
clean, good quality seed material. The objective of the present study
was to develop an efficient method for enhanced microrhizome
production in three high yielding varieties each of ginger (IISR
Mahima, IISR Rejatha and IISR Varada) and
turmeric (IISR Alleppey Supreme, IISR Prabha and
northeast cultivar Lakadong).
Culture initiation was successfully achieved using
rhizome bits with at least two viable buds on MS basal medium
supplemented with 0.1mgl-1 Kinetin and 7gl-1
agar and 15gl-1 sucrose. The cultures were multiplied in
MS medium supplemented with 0.5mgl-1 NAA, 2mgl-1
BA, 25mgl-1 Adenine sulphate, 30gl-1 sucrose
and 8gl-1 agar.
Single shoots isolated from the multiplied cultures were
inoculated and used for studying the microrhizome induction potential
under varying conditions. Almost all the cultures, irrespective of
the cultivars, grown in higher sucrose levels showed microrhizome
formation as evidenced by basal bulging of the pseudostem. Cultivar
wise differences in microrhizome induction response were observed
throughout the trials in both ginger and turmeric. In ginger
and turmeric, higher concentration of sucrose, concentration of
NH4NO3, light regime and supplementation of
Abscisic acid were conducive for microrhizome induction. The type and
size of culture vessel also influenced microrhizome induction. The
development of microrhizome in different trial media was confirmed
through visual observation of bulging of basal portion of the
pseudostem and was further confirmed using anatomical and
histochemical techniques. Micorhizomes showed all the characters of
conventional rhizome, like presence of starch grains, oil cells etc.
After three months of in vitro growth, the
microrhizome induced plants were planted out in the nursery and after
a season of growth, minirhizomes formation was achieved. Comparative
field evaluation of microrhizomes, micropropagated rhizomes,
minirhizomes and conventional rhizomes indicated superior yield
performance of minirhizomes.
The chemical constituents of microrhizomes,
micropropagated rhizomes, minirhizomes and conventional rhizomes were
compared using various phytochemical tools like Thin Layer
Chromatography, Gas Chromatography and High Performance Liquid
Chromatography and qualitative similarity could be established. The
genetic stability of microrhizome derived, micropropagated, and
minirhizome derived plants with conventional rhizome derived plants
was confirmed using RAPD markers. The pathogen free nature of
microrhizomes and micropropagated rhizomes was confirmed by in
vitro plant pathological screening using disc culture method on
Potato dextrose agar medium, Plate count agar medium and triphenyl
tetrazolium chloride medium.
In short, the microrhizome and minirhizome technologies
developed in the present study could be used for the large scale
production of genetically stable and pathogen free planting materials
in ginger and turmeric within a short period of time without
compromising the quality and quantity of the produce. These high
quality rhizomes can be used in various fields of research for the
identification, comparison and isolation of different components and
for the conservation and exchange of pure germplasm.
Tuesday, 18 August 2015
Micropropagation and conservation of Clerodendrum serratum (L.) MOON.
Micropropagation and conservation ofClerodendrum serratum (L.) MOON.
HAMZA P.V
Associate Professor,
HoD, Botany, PSMO College, Tirurangadi
2014
SUMMERY OF THE REPORT ON
UGC AIDED MINOR RESEARCH PROJECT ON Micropropagation and conservation ofClerodendrum serratum (L.) MOON
UGC AIDED MINOR RESEARCH PROJECT ON Micropropagation and conservation ofClerodendrum serratum (L.) MOON
Introduction
Clerodendrum
serratum(L.) Moon (syn. (Rotheca serrata(L,) steane & Mabb)
belong to the Family Verbenaceae is small perennial shrub found
in India, Malasia and Pakistan. It is native of of East inda
and Malasia and distributed through out India and Srilanka. It
has a long history has a source of potential chemotherapeutic agents
in various system of medicine like Ayurveda, Unani etc. It is one
of the most important plants from traditional systems of medicine
found all over the world.
Its
common name is Cheruthekku, Kurukutti (Malyalam)
Bargi
(Sanskrit)
Micro
propagation
Various
accessions of the species were collected from Botanical Garden,
Calicut University; forest of wayanad and Nilambur and southern
regions of Kerala.
Culture
were initiated from immature shoots carrying atleast single
viable bud.
Culture
initiation was conducted in basal MS medium (Murashigae and
Skoog ( 1962)
Medicinal
properties of Clerodendrum serratum are due to the presence of
major groups of chemical constituents like phenolics, flavonoids,
terpenoids and steroids. In present study three chemical
compound are taken in to consideration for the comparison of
quality of micro propagated plants to mother plant.
Results
A
total of stem accessions were collected from Kerala were planted
in the field and green house of PSMO college were established and
showed flourished growth. Culture initiation was successfully
carried out from juvenile and mature segments in basal M.S medium
The
nodal segments cut from the initiated cultures were inoculated
in to various multiplication media. All these cultures were
responded.
During
phyto chemical analysis there was no significant variations
were observed between micro propagated plant and mother plant in
their chemical compositions.
Summery
The study
can be used for large scale propagation and conservation of this
threat species and assures the resource base enhancement of the
species and there by availability of spore and pathogen free raw
materials to the pharmaceutical industry
Friday, 6 March 2015
Sunday, 8 February 2015
Why humans couldn’t
exist without plants Mohammed Raees Saidalvi
S4 Bscbotany
exist without plants Mohammed Raees Saidalvi
S4 Bscbotany
One of the
oldest forms of life on Earth are Plants. They have been part of life
much before the arrival of human beings on the planet. Man has
depended on the plants for his survival from times immemorial so much
so that plants have become an integral part of human beings. Whether
human beings or animals would be able to live on in the absence of
plants is beyond doubt.
The disappearance of plants from the
earth would be disastrous to both people and also animals. Actually
it would be worse than any other natural calamity or even manmade
nuclear threat. Bigger animals are dependent on smaller animals which
in turn have plants for their food. People may find solutions or make
other arrangements to problems arising from the non availability of
plants, but the required quantity of food and oxygen both for human
and animal consumption would be impossible to replace. So there
wouldn't be any people or even animals
without plants. An earth without
plants means a planet without animals and people. To protest for
ourselves we have to protest for plants!
Subscribe to:
Comments (Atom)